
Copyright © Mauve Technology Limited 2013
malvaSAFE technology creates instant, on-demand, ultra-effective biocidal solutions
with fast contact times at room temperatures without the need for expensive, generator-type,
capital equipment.
Our technology permits us to simply mix two separate solutions - one chlorine-donating
and one pH modifying. The resultant dilute biocide is produced instantaneously at
a near neutral pH [6-7], resulting in a huge release of Hypochlorous Acid [HOCl]
replicating the human body’s own defensive biocide.
It is our precise control of the final solution’s pH that is responsible for the efficacy of our disinfecting malvaSAFE technology.
Our component chemicals can be mixed together in either dilute form or can be added as twin concentrates to a larger volume of water to create the desired disinfectant concentration.
The concentration can be tailored to suit any application - low strengths for water
purification, and higher strengths for example for medical equipment sterilisation,
where higher levels of the active component, Hypochlorous Acid, are required.
Solution concentration strength is often measured as Free Available Chlorine [FAC] measured as parts per million [ppm]. The higher the strength in ppm, the more effective the biocidal effect of the solution.
Free Available Chlorine [FAC] is chlorine that is present in the form of HOCl, OCl-
Secondary applications of our malvaSAFE technology can utilize either a water-
There are no other disinfecting systems that are able to produce such large amounts
of HOCl, on demand & with no expensive capital equipment such as a brine-
The technical bit -
Chlorine in water splits into two distinct forms, or species. Hypochlorous Acid [HOCl]
& Hypochlorite ion [OCl-
According to most studies HOCl is around 100 times more effective as a biocide than
OCl-
For example, HOCl requires by far the shortest contact time to achieve a 99% kill of E. Coli.
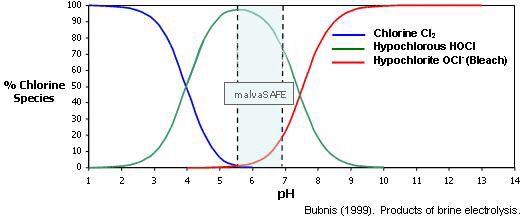
How much of these two types are present is totally dependent upon the pH of the final solution, please see Fig. 1. for a visual explanation.
Fig. 1. Effect of the solution pH on formation of different Chlorine species.
As pH rises [above 7.5] less HOCl & more OCL-
malvaSAFE solutions are always produced in a pH range [6 – 7] designed to optimise
the production of HOCl and maximize their biocidal efficacy. For example at a pH
of 6.5 about 90% of FAC is in the form of HOCl with about 10% as OCl-
HOCl is electrically neutral whereas Hypochlorite ions are electrically negative; as such, both substances have very distinctive, and different, behaviours.
Cell walls of pathogenic microorganisms are also negatively charged; this means that
they can only be penetrated by the neutral HOCl molecules. Besides its neutrality,
HOCl is much more reactive and a much stronger disinfectant than OCl-
The HOCl solution produced is at near neutral pH (between 5 and 7), optimizing its biocidal efficacy, yet remaining completely safe to humans and the environment.
An additional benefit of the efficacy of HOCl is the fact that in typical applications,
such as endoscopy, the strength of the malvaSAFE solutions need only be 200 ppm,
making them non-
An added benefit of malvaSAFE is that after use, no special disposal protocols are required.
malvaSAFE - the technology
| malvaSAFE |
| malvaSEE |
| malvaSAFE technology |
| malvaSAFE performance |
| malvaSAFE why switch? |
| malvaSAFE products |
| malvaSAFE commercial opportunities |